Clean Room Validation
AFi validation service provides the environmental conditions of the clean rooms that have been defined in the HVAC order from the user process requirements. Cleanroom and equipment’s are a major capital investment for you to get contamination control environment, regular testing helps you to know whether investment is operation properly. Regular validation minimizes product defects, costly downtime and hence increases productivity. AFi validation team is providing independent customer friendly cleanroom validation and cleanroom certification service with sophisticated calibrated instruments.
The air quality in clean rooms and clean air devices is critical for optimum working of processes and typically used in manufacturing, packaging, and research facilities associated with industries like pharmaceuticals, semiconductor, aerospace, research facilities, laser and optic industries etc.
When a clean room has just been built it must be tested to ensure that it is meeting the regulatory requirements given in the ISO 14644-1. Validation of a new cleanroom follows a specified lifecycle. The life cycle comprises five phases each of which accomplishes particular tasks to control variation in the modular environment. It starts off with the design control phase and ends with monitor and control. Changes to equipment and control factors after the cleanroom has been validated are grounds for cleanroom re-validation.
Our Qualified clean room performance testing (CPT) ensures the following tests as part of it tests and validation program:
- Airflow Velocity and Uniformity Tests
- HEPA Filter Installation Leak Tests
- Room Particle Count Tests
- Enclosure Pressurization Tests
- Particle Fallout Count Tests
- Containment Test
- Air Exchange Rate
(*All our validation instruments are accurately calibrated once every year on a scheduled date)
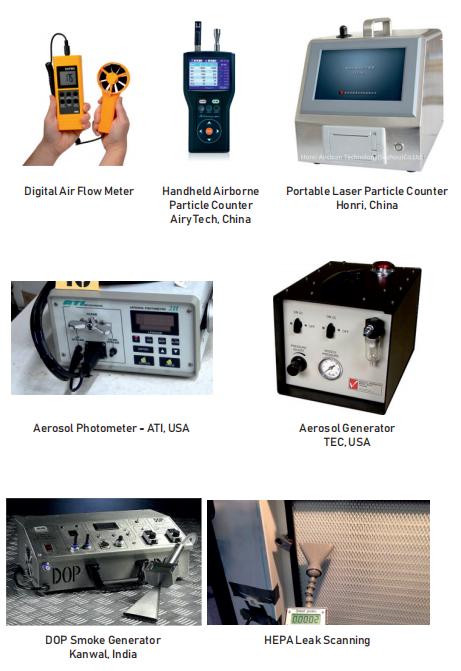
AFi are well equipped with latest sophisticated imported clean room validation instruments: